Introduction: Patients (pts) with triple-class exposed (TCE) relapsed/refractory multiple myeloma (RRMM) represent a particularly difficult-to-treat population. A pooled analysis of the prospective, non-interventional, multinational LocoMMotion (NCT04035226) and MoMMent (NCT05160584) studies previously demonstrated suboptimal outcomes in pts with TCE RRMM. Extramedullary disease (EMD) is an aggressive feature of multiple myeloma characterized by the presence of plasmacytomas that have spread beyond the bone marrow. A recent systematic literature review (Bladé et al, 2022) found that the prognosis of pts with EMD is poor, as most current treatment (tx) options are notably less efficacious in pts with EMD than in those without EMD. The review also found that there are few prospective studies evaluating tx approaches in pts with EMD. Additional prospective data are needed to better understand current txs and outcomes for pts with EMD. In this analysis, we describe current txs and outcomes in pts with TCE RRMM with EMD over the past 3 years in LocoMMotion and MoMMent.
Methods: Data were pooled from LocoMMotion (median follow-up [mFU], 26.4 months [mo; range, 0.1-35.0]; enrolled Aug 2019-Oct 2020; N=248) and MoMMent (mFU, 9.3 mo [range, 0.4-15.3]; enrolled Nov 2021-Jul 2022; N=54). Both studies have similar designs; eligible pts received ≥3 prior lines of therapy (LOT; LocoMMotion allowed <3 prior LOT if pts were double refractory to a proteasome inhibitor and immunomodulatory drug), were TCE, had measurable disease and documented progressive disease since their last LOT, and had an ECOG performance status (PS) of 0 or 1 at screening. EMD was defined as extramedullary plasmacytoma reported by the investigator. The assessment of plasmacytoma was not mandated but, per protocol, it was anticipated that extramedullary plasmacytomas would be prospectively assessed by clinical examination or radiologic imaging at baseline (BL) for all pts with a history of plasmacytomas, or if clinically indicated prior to the first dose of standard-of-care (SOC) therapy. If an assessment was not performed, the pt was considered not to have BL plasmacytoma. Responses were evaluated per IMWG criteria by a response review committee. A Cox proportional hazards model and Kaplan-Meier methodology were used to estimate medians, 95% CIs, and survival curves (unadjusted and adjusted for potential confounders) for progression-free survival (PFS) and overall survival (OS).
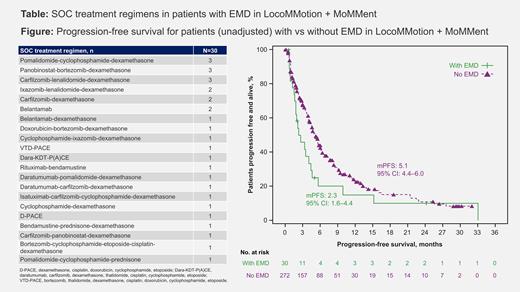
Results: The pooled analysis included 302 pts with mFU of 24.3 mo (95% CI, 21.0-25.6; data cut-off, Oct 27, 2022 [LocoMMotion], Mar 13, 2023 [MoMMent]). 30 pts had EMD at BL and 272 did not. Anatomical locations of plasmacytomas in these pts included vertebra, liver, pelvis, skin, and lymph nodes, suggesting a mix of purely extramedullary (harder to treat) and bone-related plasmacytomas. Duration of study follow-up was similar for pts with EMD (26.5 mo [95% CI 17.3-31.9]) and those without (24.3 mo [95% CI 20.3-25.6]). BL demographics and disease characteristics were generally comparable, with ≥10% difference in ISS stage III, LDH >245 U/L, and ECOG PS of 1 observed in pts with EMD. A greater proportion of pts with EMD than those without EMD were penta-drug exposed (56.7% vs 45.2%) and had received prior autologous stem cell transplant (76.7% vs 62.5%). Pts with EMD received 21 unique SOC tx regimens, primarily chemotherapy based (Table). The ORR was lower for pts who had EMD vs those who did not (7/30; 23.3% [95% CI 9.9-42.3] vs 89/272; 32.7% [27.2-38.6]). Pts with EMD also had a shorter median PFS (2.3 mo [95% CI 1.6-4.4] vs 5.1 mo [4.4-6.0]) and median OS (7.2 mo [95% CI 5.4-18.6] vs 15.1 mo [12.0-18.5]) compared with those without EMD (Figure). The adjusted survival curves have similar patterns to the unadjusted. Among the 30 pts with EMD, there were 23 and 21 PFS and OS events, respectively; conclusions based on time-to-event data should therefore be interpreted with caution due to a small sample size.
Conclusions: These results indicate that there is a significant unmet medical need for better tx options in this population of pts with TCE RRMM, specifically in pts with EMD. This need is illustrated by the poor outcomes observed with existing txs and the absence of an effective established SOC for this condition. Conducting clinical trials with innovative therapies, using clearly defined criteria for EMD, is essential to address the unmet medical needs of these pts.
Disclosures
Moreau:GSK: Honoraria, Other: Advisory Board; janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards. Mateos:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Takeda: Honoraria; Regeneron: Honoraria. Goldschmidt:Mundipharma: Research Funding; BMS/Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Array Biopharma: Research Funding; Johns Hopkins University: Research Funding; Dietmar-Hopp-Foundation: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Chugai: Honoraria, Patents & Royalties, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; Incyte: Research Funding; Heidelberg Pharma: Research Funding; KaryoPharm: Research Funding; Hoffman- La Roche: Research Funding; Glycomimetics: Research Funding; GSK: Honoraria, Other: Travel Support, Research Funding; Millenium Pharmaceuticals: Research Funding; Molecular Partners: Research Funding; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; MSD: Research Funding; Morphosys AG: Research Funding; Pfizer: Honoraria, Patents & Royalties: Travel Support, Research Funding; Takeda: Research Funding; Novartis: Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnology: Membership on an entity's Board of Directors or advisory committees. Besemer:Janssen Cilag: Honoraria; GSK: Honoraria. Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding. Lindsey-Hill:AbbVie: Consultancy. Delforge:Sanofi: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Bristol Myers Squibb: Consultancy, Speakers Bureau; Stemline: Consultancy, Speakers Bureau. Angelucci:BMS, Vertex: Other: participation in DMC; bluebird bio, GSK: Membership on an entity's Board of Directors or advisory committees, Other: involvement w/ advisory boards; Novartis: Honoraria; Menarini/Stemline: Consultancy. Vij:Takeda: Honoraria, Research Funding; Karyopharm: Honoraria; Harpoon: Honoraria; Legend: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding. Doyle:Janssen: Current Employment, Current equity holder in publicly-traded company. Gray:Janssen: Current Employment, Current equity holder in publicly-traded company. Albrecht:JANSSEN: Current Employment. Strulev:Janssen Pharmaceutica NV: Current Employment. Haddad:Janssen Cilag France: Current Employment. Saarinen:Janssen: Current Employment. Mitchell:J&J employee, Statistician: Current Employment, Current holder of stock options in a privately-held company, Honoraria. Buyze:Janssen Pharmaceutica: Current Employment. Weisel:Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Other: Research grant to institution; Adaptive Biotech: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Research grant to institution; AstraZeneca: Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Other: Research grant to institution; Oncopeptides: Consultancy, Honoraria; Novartis: Honoraria; Roche Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other: Research grant; Sanofi: Consultancy, Honoraria, Other: Research grant to institution; Menarini: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Stemline: Honoraria; Amgen: Consultancy, Honoraria, Other: Research grant to institution; AbbVie: Consultancy, Honoraria, Other: Research grant to institution.